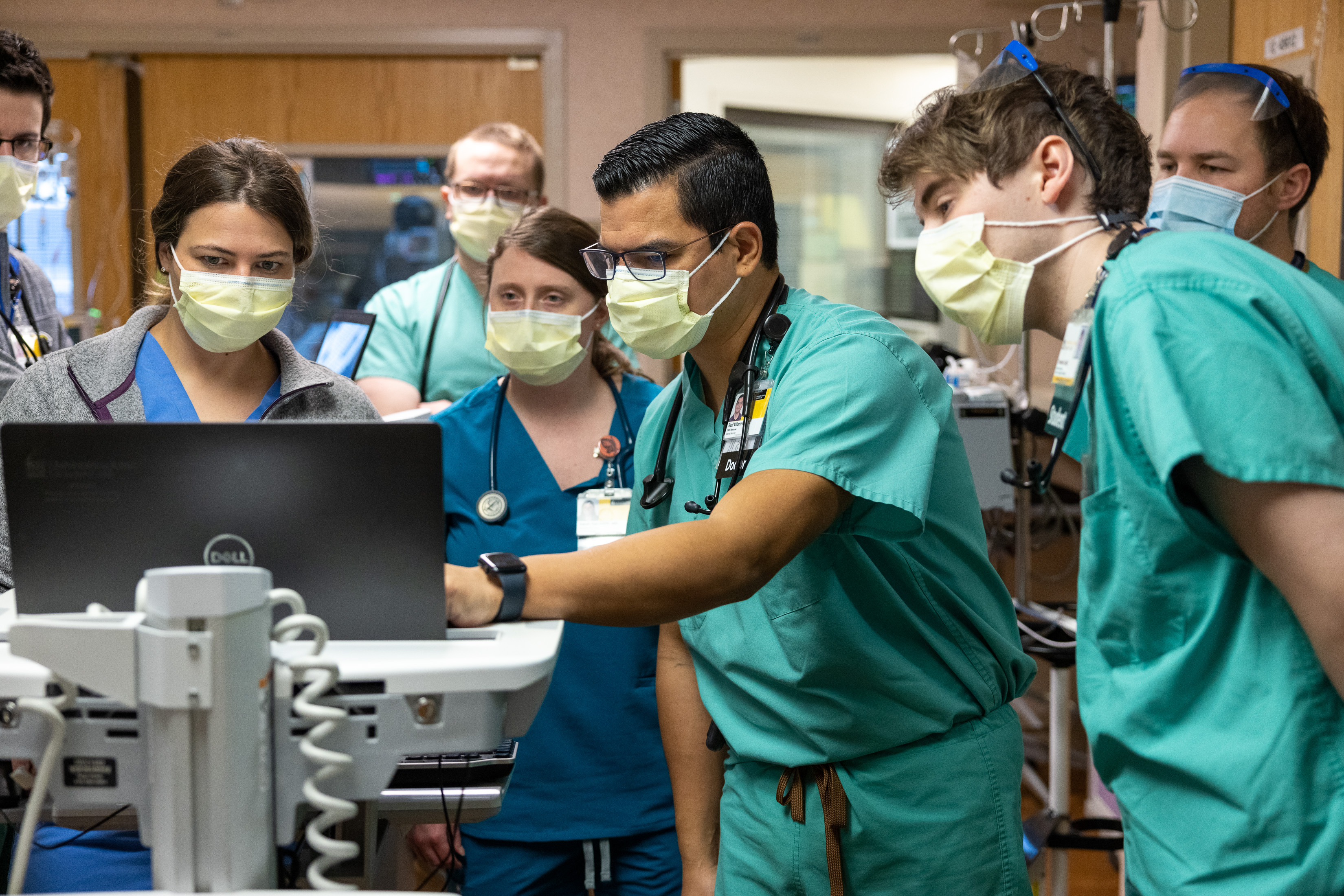
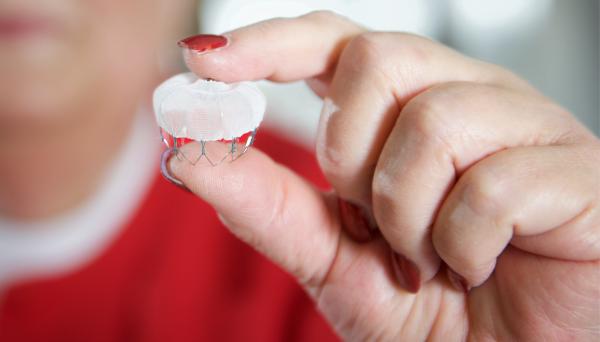
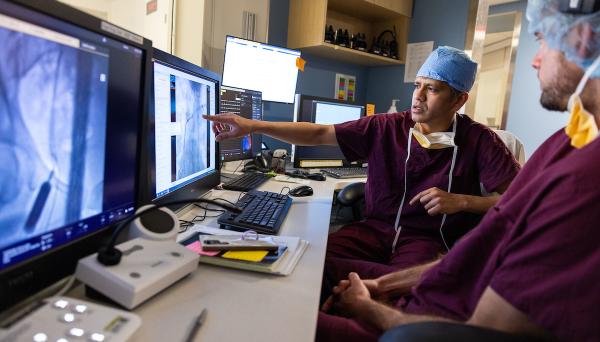
Standard safety measures for visitors, face masks now in place
April 11, 2024
Marion swimmer back in the water after care with UI Sports Medicine
April 8, 2024
UI helping Iowa’s community hospitals improve cancer care
March 26, 2024
Spine surgery helps young golfer get back in the swing
March 25, 2024